You’re here because your customer has made it a requirement for your company to do traceability. For certain foods, traceability is also a requirement of the law under the U.S. Food and Drug Association (FDA) Food Safety Modernization Action Section 204(d) or FSMA 204.
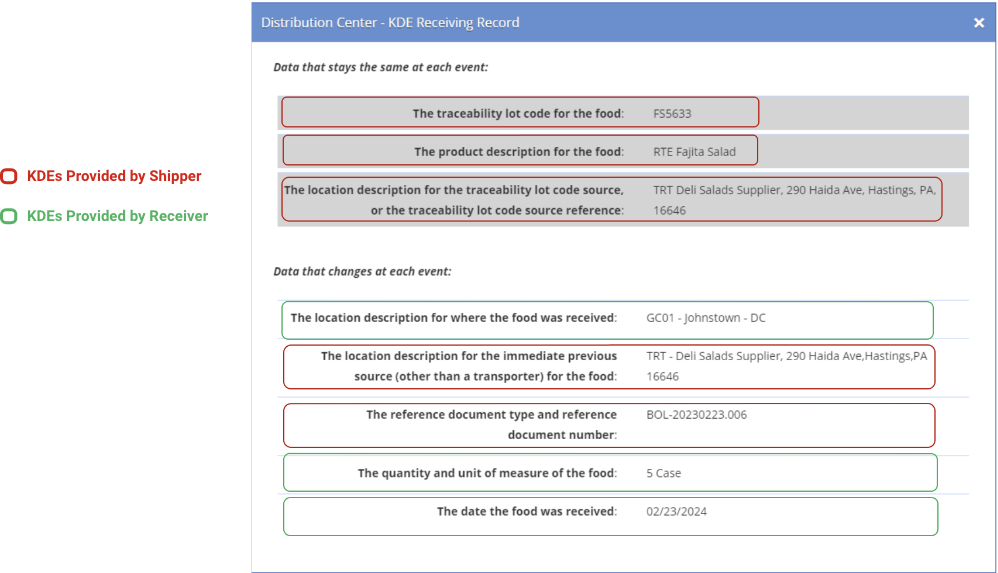
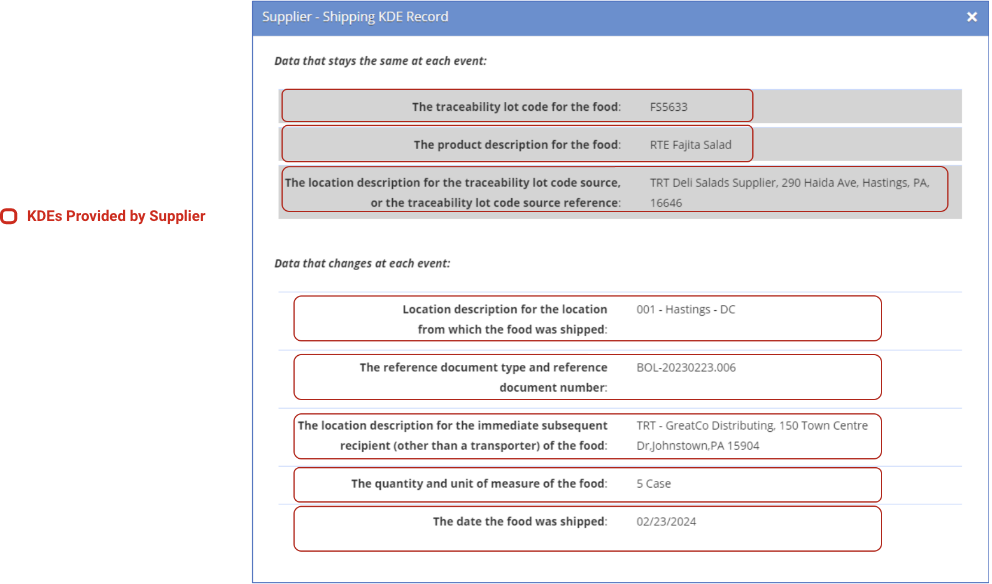
The ReposiTrak Traceability Network® enables the fast, easy exchange of Traceability Lot Codes and other traceability information known as Key Data Elements (KDEs), the term used by the FDA to describe the specific pieces of data used to track the path of products through the supply chain.
Once connected to the ReposiTrak Traceability Network, suppliers can share traceability information with an unlimited number of in-network retail and wholesale customers to meet their traceability requirements.
To get connected, simply follow the link you received in the email invitation from ReposiTrak on behalf of your customer. The “Get Started” link in that email will take you to a self-guided, step-by-step process where we’ll gather information, help you to create a “test” file and also choose a way to send us your traceability file for every shipment. If you have questions or lost your link, please contact support@repositrak.com.
Below, is more information about the different ways you can share your traceability data with ReposiTrak. In the tabs to the right, you’ll find detail about each required KDE, the various communications protocols that you can use, FAQs, and more.
KDEs need to be shared through the ReposiTrak Traceability Network every time you ship your product to your customer. There are three ways you can get this information to us:
- Fully Automated – If you already have a working system that contains your traceability data, then you can set up an automated export directly from that system to ReposiTrak. We’ll accept files in any format including Excel (XLSX), Comma Separated Values (CSV) and Electronic Data Interchange (EDI). You’ll also be provided with options for how to send your files to us automatically. You can explore the different communications protocols on tab 3. CHOOSE YOUR DATA SHARING PROTOCOL. The most common protocols we see are FTP, SFTP, AS2 and SMTP email.
- Semi-Automated – There are two ways that you can semi-automate how you share your traceability data with ReposiTrak for every shipment:
- Automated export/manual send: If you have a way to automatically export the data from your systems, but not a way to automatically send it to us, choose this method. In tab 2. CREATE TRACEABILITY FILE, you’ll find Excel and CSV templates that you can use as a model for your file exports. Once you have your files set up, you can then manually email the file to ReposiTrak every time a shipment is sent OR upload your file online within your ReposiTrak account.
- Manual export/automated sent: We also provide options for suppliers who need to pull their data manually, but have a way to automatically send files to ReposiTrak. In tab 2. CREATE TRACEABILITY FILE, you’ll find Excel and CSV templates that you can use as a model for your files. Then, on your side, you can place file you created in a directory on your network that is setup to automatically send the file through either FTP, SFTP or SMTP email to ReposiTrak.
- Manual – Manual data entry processes are possible, but have a greater risk of errors due to typos and a greater risk of missing information due to the time it takes to manually key in and submit data each time a shipment is sent.
- If you choose to enter your traceability data manually, use the Excel template to create a file containing the items you typically sell to your customer. Then, edit the traceability data that change each time a shipment is sent to that customer. You can then manually email to us, or upload it online within your ReposiTrak account.
- There is also an option to manually key in your traceability data within your ReposiTrak account using our easy-to-use shipment screens. View the Training Guide | Entering a Shipment Online, which can also be found on the TRAINING GUIDES tab.